CAS:1051375-16-6
1051375-16-6
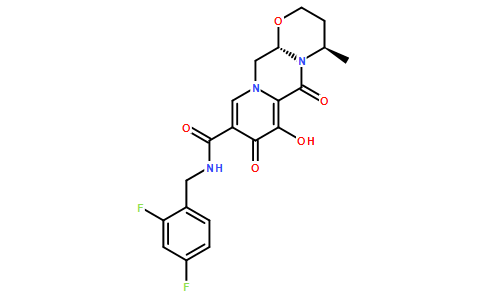
CAS号:1051375-16-6
品名:度鲁特韦Dolutegravir
中文别名:度鲁特韦
英文别名:(4R,12aS)-N-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide;(4R,12aS)-N-[(2,4-Difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide;(4R,12aS)-N-[(2,4-difluorophenyl)methyl]-7-hydroxy-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2H-pyrido[5,6]pyrazino[2,6-b][1,3]oxazine-9-carboxamide;GSK1349572;S/GSK1349572;Tivicay;
分子式:C20H19F2N3O5
分子量:419.379
精确质量:419.129
Psa:100.87
MDL号:MFCD20488027
外观与性状:白色或淡黄色固体
密度:
熔点:188-192°C
简介:Dolutegravir(DTG)isanFDA-approveddrugforthetreatmentofHIVinfection.Dolutegravirisanintegraseinhibitor.KnownasS/GSK1349572orjust\"572\"thedrugismarketedasTivicaybyGlaxoSmithKline(GSK).InFebruary,2013theFoodandDrugAdministrationannouncedthatitwouldfasttrackdolutegravir'sapprovalprocess.OnAugust13,2013,dolutegravirwasapprovedbytheFDA.OnNovember4,2013,dolutegravirwasapprovedbyHealthCanada.OnJanuary16,2014,TivicaywasapprovedbytheEuropeanCommissionforusethroughouttheEuropeanUnion.
微生物Microbiology